Specifications
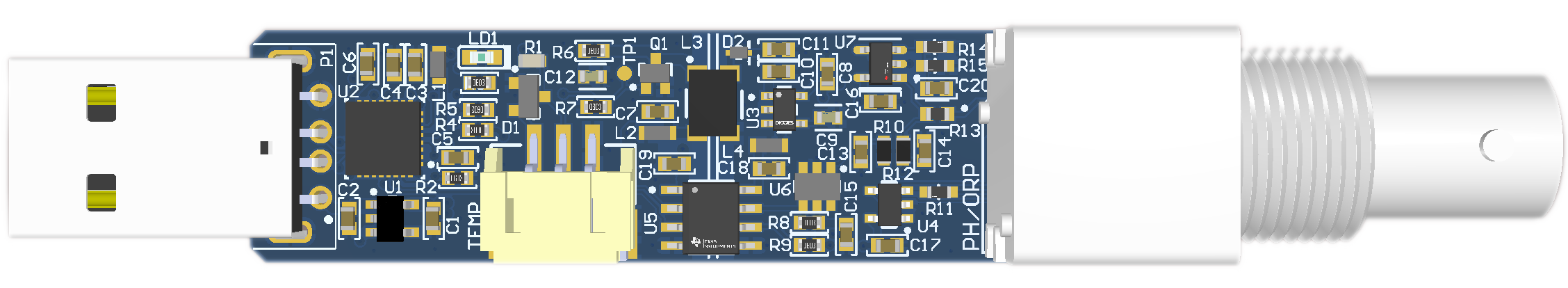
Specifications¶
- Output format: JSON, CSV or Human readable
- Host compatibility: Linux, MacOS, Windows and Android systems (driver integrated in most OS)
- Data reporting rate: configurable between 1 second and 1 hour
- Calibration: 2-points, automatic buffer detection
- Automatic Temperature Compensation: Yes. From on-board or external temperature probe
- Measuring Range:
- pH: 0 to 14.00 pH
- ORP: ±1,650 mV
- Sensitivity:
- ADC: 4uV (pH range), 16 uV (ORP range)
- Reported output: 0.001 pH, 0.1 mV (ORP)
- Stability: 0.01 pH or 1 mV per 24 hours, non-cumulative (*1)
- Input Impedance: > 1 TΩ (1x1015 Ω)
- Input Bias current: 20 fA (typical)
- Temperature resolution: 0.0625°C
- ADC resolution: 18-bit
- Power input: 5V from USB port / 23 mA (115 mW)
- Operating range: -40 <‒> +85 °C, 10 <‒> 90 %r.H.
- Dimensions: 95x16x20.5 mm
- Weight: 17.6 g
Output data points:
- pH (calibrated and temperature compensated) - last measurement
- pH (calibrated and temperature compensated) - average
- Raw Voltage [mV] - last sampling period
- Raw Voltage [mV] - average
- Temperature - on-board sensor
- Temperature - external probe
Dimensions¶
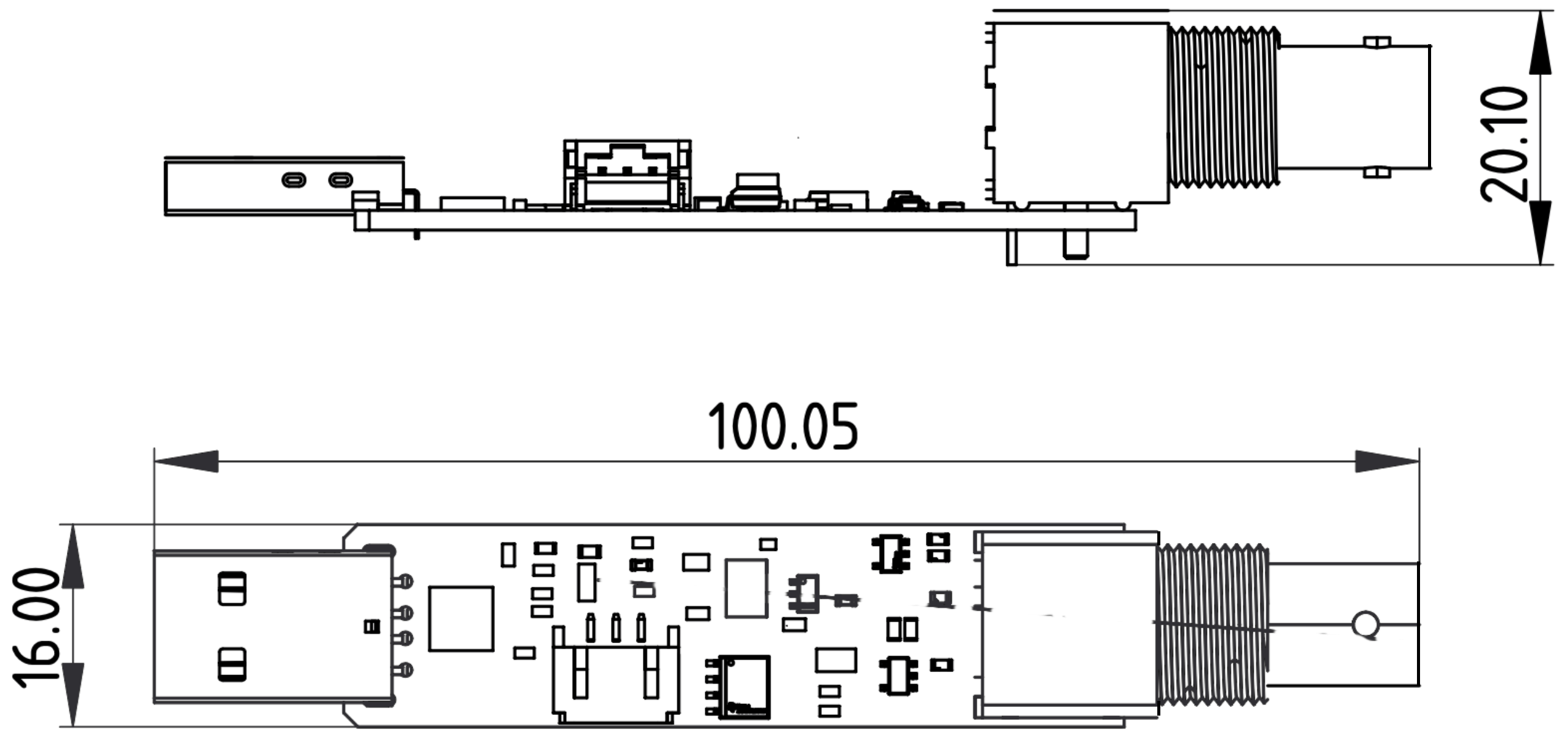 uThing::iPH dimensions [mm]
uThing::iPH dimensions [mm]
Notes¶
(*1) The stated stability is for the measurement unit (voltage acquisition). When measuring pH/ORP the values can drift over time due to several reasons:
For instance, it may take 10 to 20 minutes for the sensor temperatures to equilibrate (electrode and temperature probe).
The sensor voltage depends on the pH of the liquid at the surface of the pH sensitive glass. If the bulb is dirty or fouled, it may take some time for the liquid to diffuse through the coating and reach the membrane. The response of the sensor to step changes will be slow, and readings will drift to the final values.
Memory of past junction potentials can also lead to drift. When a pH sensor is transferred from one sample to another, liquid that has diffused into the reference junction is carried with the sensor. When the sensor is first placed in the new solution, both the old and new solutions determine the junction potential. As the new solution diffuses into the junction and the old solution diffuses out, the liquid junction potential gradually changes. The changing junction potential causes the pH reading to drift. The effect is most severe when the ions involved have substantially different mobilities. If the solutions are more or less equitransferent, the memory effect will be relatively small. The type of junction also influences the amount of drift. In flowing junctions, the filling solution washes out the previous sample, so the time for the memory to disappear is a few minutes to an hour. In gel type sensors, diffusion alone is responsible for movement of ions through the junction. Therefore, the time required for the memory to dissipate can be quite long.