pH measurement¶

Definition¶
pH measurement is used in a wide variety of applications: agriculture, wastewater treatment, industrial processes, environmental monitoring, and in research and development.
A standard pH measuring system consists of three elements:
- pH electrode (pH probe)
- temperature compensation element (temperature probe)
- pH meter or controller
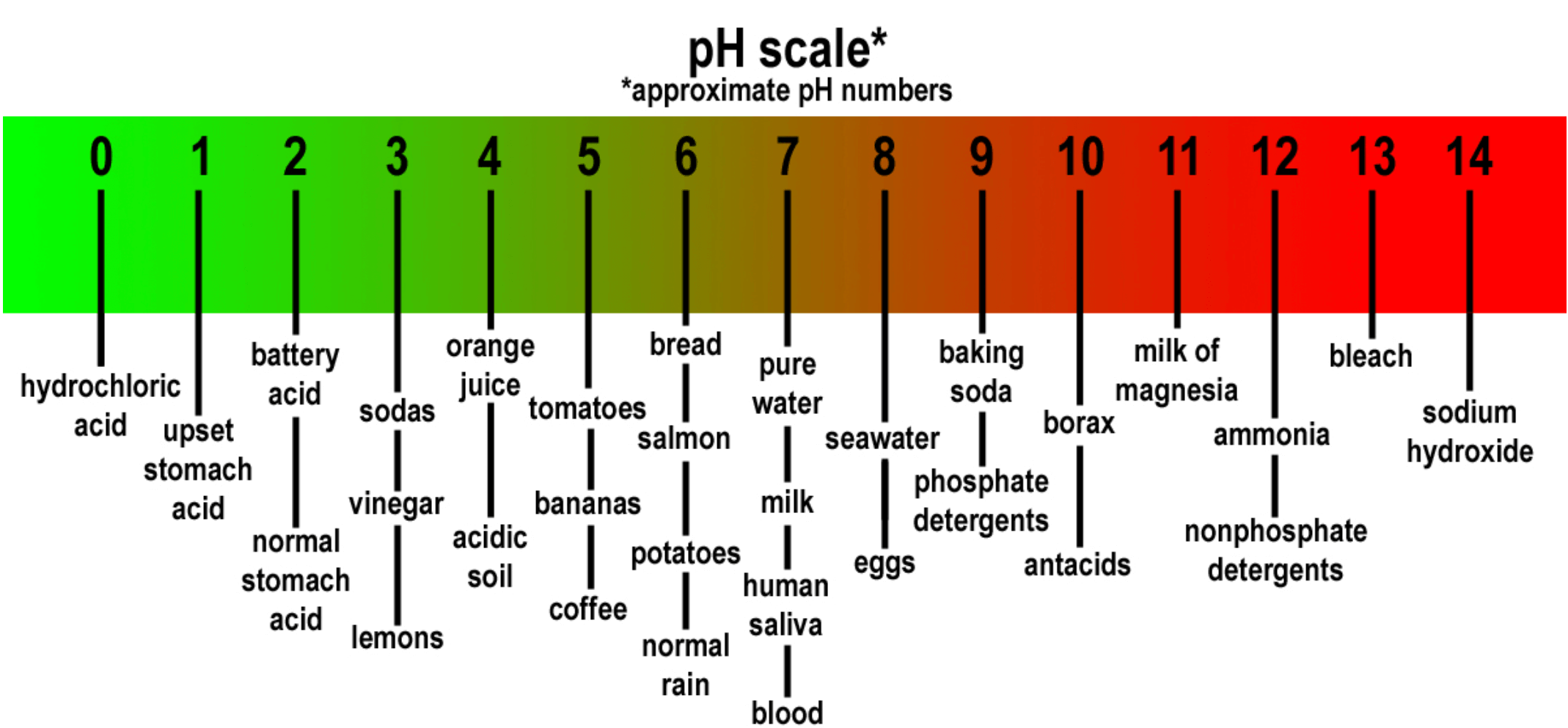
pH is a measure of the acidity or alkalinity of a solution. The pH value states the relative quantity of hydrogen ions (H+) contained in a solution.
The greater the concentration of H+ the more acidic the solution and the lower the pH. In this relationship, pH is defined as the negative logarithm of hydrogen activity.
Most pH readings range from 0 to 14. Solutions with a higher [H+] than water (pH less than 7) are acidic. Solutions with a lower [H+] than water (pH greater than 7) are basic or alkaline.
Specifically, the pH value of a solution is the negative logarithm of its hydrogen ion activity (α), which is the product of hydrogen ion concentration [H+] and the activity coefficient of hydrogen (γH+) at that concentration:
pH electrodes¶
Construction¶
The modern pH electrode is a combination electrode composed of two main parts: a glass electrode and a reference electrode as shown in the figure below:
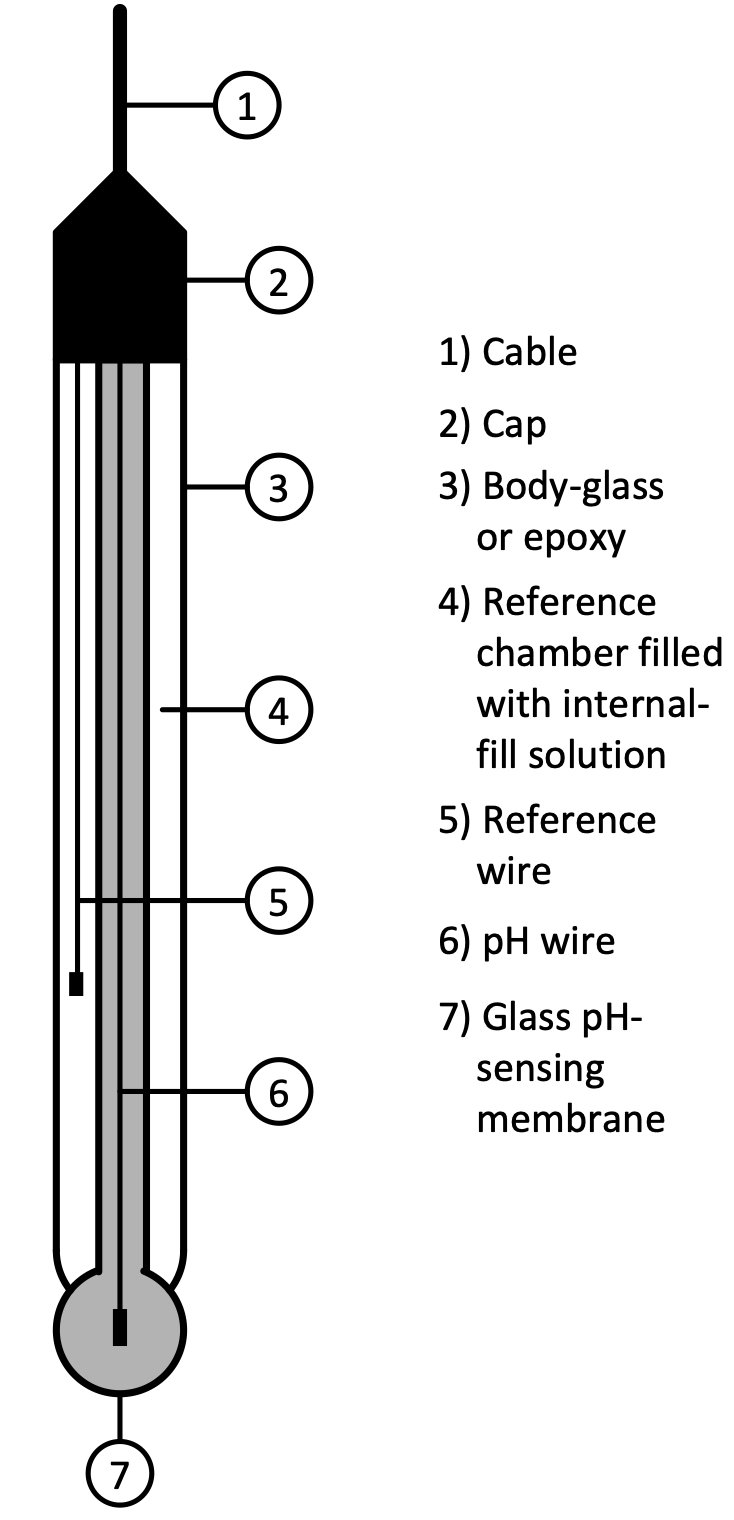
pH is determined essentially by measuring the difference of potential (voltage) between these two electrodes.
At the tip of the electrode is the thin membrane that is a specific type of glass that is capable of ion exchange. It is this element that senses the hydrogen ion concentration of the test solution.
The reference electrode potential is constant and is produced by the reference electrode internal element in contact with the reference fill solution, often potassium chloride (KCl) and silver chloride (AgCl2) that is kept at a pH of seven.
The source impedance of a pH electrode is very high because the thin glass bulb has a large resistance that is typically in the range of 10 MΩ to 1000 MΩ. This means that the electrode can only be monitored by a high-impedance measuring device.
The uThing::iPH™ analog frontend was carefully designed to have an input bias current as low as 20 fA!, or an equivalent input impedance well over the TeraOhm range, achieving insignificant offset error even with the most demanding probes (i.e. ±0.2 mV for a 1 GΩ probe).
Transfer function¶
The transfer function of the pH electrode is:
where:
- pH(X) = pH of unknown solution(X)
- pH(S)= pH of standard solution = 7
- ES = Electric potential at reference or standard electrode
- EX = Electric potential at pH-measuring electrode
- F is the Faraday constant = 9.648*104 [C / mol]
- R is the universal gas constant = 8.314 [J / K mol]
- T is the temperature in Kelvin
From the transfer function, can be seen that as the pH of the solution increases, the voltage produced by the pH-measuring electrode decreases.
Temperature dependence¶
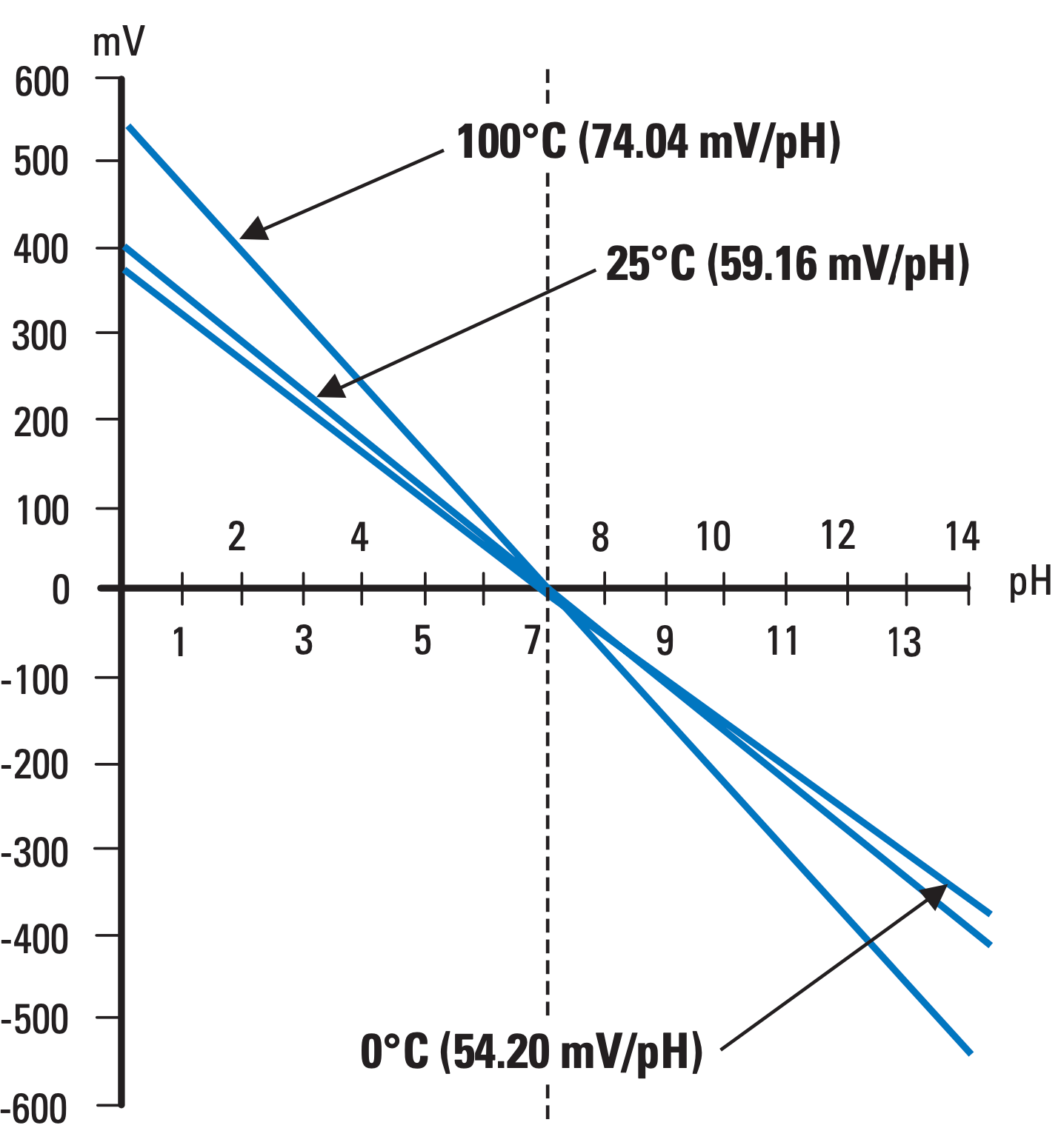
It is important to note that a pH electrode’s sensitivity varies over temperature. Looking at the pH-electrode transfer function shows that the sensitivity linearly increases with temperature according to the following equation.
Temperature dependence
For example at 25°C, electrode sensitivity is 59.16 mV/pH and the output of the electrode will swing from +414.12 mV (pH 0 strong acid) to -414.12 mV (pH 14 strong base).
However, if the measured solution temperature is increased to 100°C, the output will swing from +518.29 mV down to -518.29 mV.
Due to this behavior, it is critical to know the temperature of the solution being measured and compensate the measurement accordingly.
Automatic Temperature Compensation¶
The uThing::iPH™ performs continuous "Automatic Temperature Compensation (ATC)" on the measured probe's voltage. The dongle can use two sources for the compensation temperature:
On-board temperature sensor¶
The dongles integrates an on-board temperature sensor calibrated to measure ambient temperature.
Dongle temperature
The on-board sensor is calibrated at ambient temperature during manufacturing with the dongle connected through an USB extension cable. In cases where the calibration and the online process to measure are at similar ambient temperatures, this sensor provides acceptable temperature correction (pH measurement error relatively small) providing that the dongle is connected to the host through an USB extension (if the dongle is directly connected to a host, the heating transfered from the host will affect the dongle's temperature, diverting from ambient and the liquid to measure, thus increasing the pH error).
This is the default reference when no external temperature probe is connected.
External temperature probe¶
For the most accurate pH measurements, specially when the temperature of the liquid to measure can divert from the ambient temperature, the dongle needs the exact temperature of the liquid.
Therefore, it's recommended to use the external isolated, waterproof DS18B20 temperature probe (sold separately)

When the external probe is detected, the ATC algorithm will use this temperature as the reference.
BNC connector¶
The dongle uses a BNC connector, the most widely used on the industry, making the dongle compatible with a wide range of consumer and lab-grade pH and ORP probes.
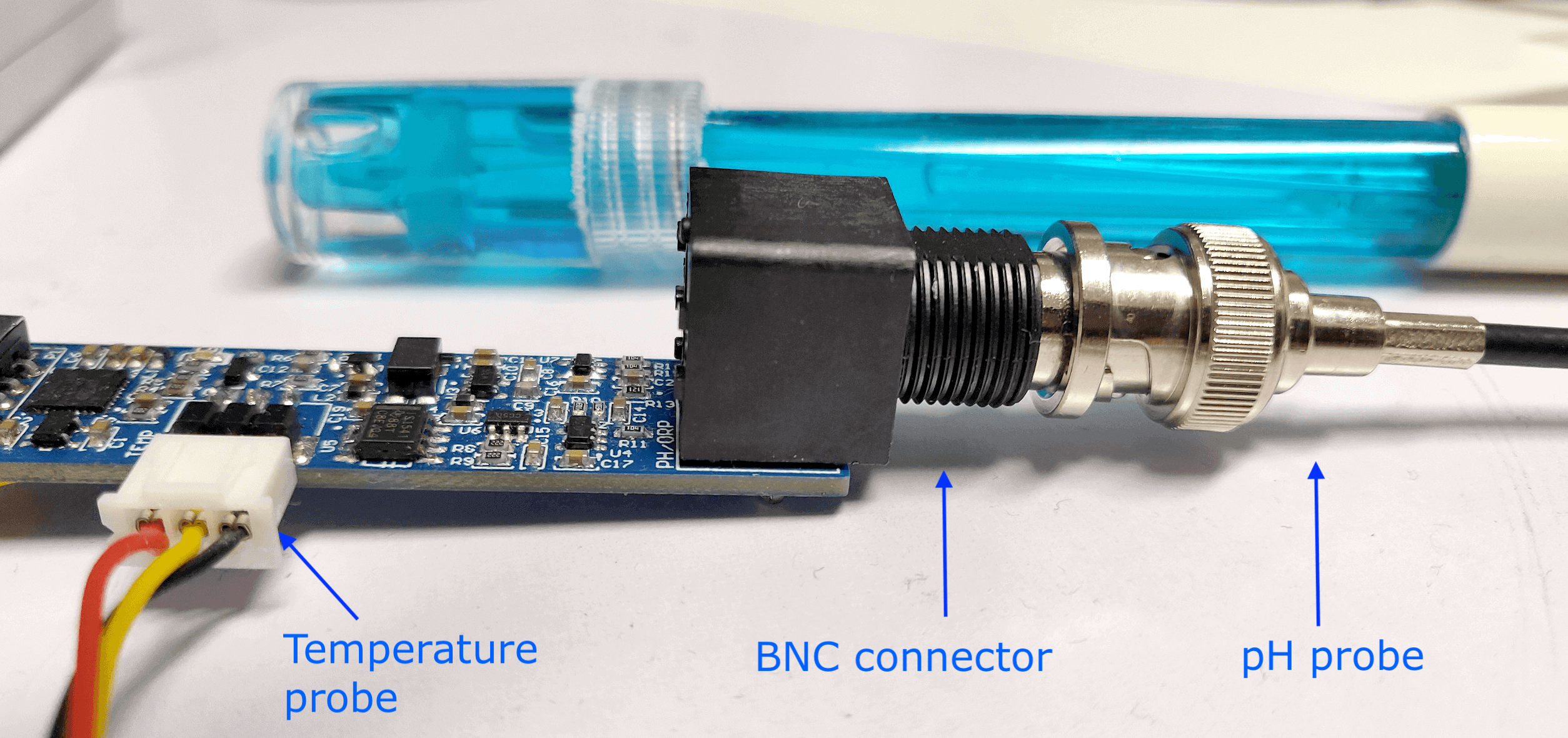 BNC connector and probes
BNC connector and probes
Probe calibration¶
Ideal vs Real probes
An ideal pH electrode will output exactly 0 mV at pH = 7.00, and present a slope of -59.16 mV/pH at 25ºC. As with any sensor, real pH probes will always differ from the ideal response, and their response will change over time due to degradation of the glass interface, contamination, and other effects. Therefore, in order to ensure accurate, repeatable measurements, it's important to perform regular calibrations of the probe.
In order to produce accurate and repeatable measurements, a calibration procedure needs to be performed every time that a new probe is used with the meter.
In addition, since the probe response changes over time due to degradation of the glass interface, contamination (coating), and other effects, regular calibrations are recommended.
The frequency of calibration will vastly depend on the application and performance of the electrode. In some cases, the probe's manufacturer will recommend a calibration period.
In water-quality / waste-water applications, a monthly calibration might be sufficient. In applications with extreme pH levels (very acidic or alcaline solutions), more frequent calibration might be needed.
Verifying calibration state
As a simple way to quickly verify if your pH meter currently needs calibration, dip the pH probe into a calibration buffer and check if the measurements have a big deviation to the buffer's value. For most applications, if the difference is within 0.05 pH, calibration is not needed.
Please refer to the calibration section for details on the procedure.